Abstract
Introduction: In the pivotal ZUMA-5 trial, axicabtagene ciloleucel (axi-cel; an autologous anti-CD19 chimeric antigen receptor T-cell therapy) demonstrated high rates of durable response in r/r FL patients. When ZUMA-5 24-month data were compared to SCHOLAR-5, clinically significant benefits in overall response rate (ORR) and survival outcomes were shown in patients treated with axi-cel (ASH 2021, Palomba et al., abstract 3543). The aim of this study was to compare clinical outcomes from updated 36-month ZUMA-5 to a weighted sample from the SCHOLAR-5 cohort.
Methods: The international SCHOLAR-5 external control cohort consists of r/r FL patients from 7 institutions in 5 countries who initiated a third or higher (3L+) line of therapy (LOT) after July 2014 as well as patients from the pivotal DELTA trial initiating treatment following idelalisib. ZUMA-5 trial eligibility criteria were applied to the SCHOLAR-5 cohort with patients excluded or censored upon transformation. The SCHOLAR-5 and ZUMA-5 cohorts were balanced for patient characteristics through propensity scoring on prespecified prognostic factors and standardized mortality ratio weighting. Characteristics with a standardized mean difference (SMD) <0.1 were deemed balanced. Overall survival (OS), progression-free survival (PFS) and time-to-next treatment (TTNT) were evaluated using Kaplan-Meier analysis.
Results: 143 patients were identified in SCHOLAR-5, reducing to 129 patients after applying propensity score weights versus 127 patients in ZUMA-5. Median follow-up time for ZUMA-5 and SCHOLAR-5 were 36.8 and 26.2 months, respectively. Variables that were successfully balanced (SMD<0.1) included POD24, number of prior LOT, relapsed vs refractory, prior stem cell transplant, size of largest nodal mass, response to prior LOT, time since last therapy, and age.
ORR was 54.0% (69/129) in SCHOLAR-5 compared to 93.7% (119/127) in ZUMA-5. CR was 45/129 (34.9%) in SCHOLAR-5 compared to 100/127 (78.7%) in ZUMA-5.
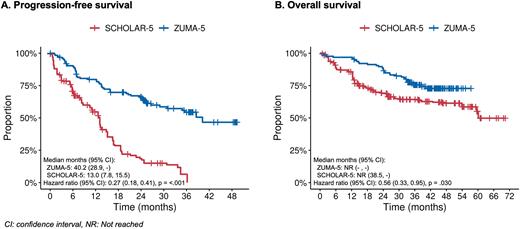
The median PFS was 13.0 months in SCHOLAR-5 compared to 40.2 months in ZUMA-5 (Figure 1a). Median TTNT was 26.5 months in SCHOLAR-5 and was not reached in ZUMA-5. Median OS was not reached in SCHOLAR-5 or ZUMA-5 (Figure 1b). The hazard ratios for PFS, TTNT and OS were 0.27 (95%CI: 0.18-0.41), 0.60 (95%CI: 0.39 - 0.93), and 0.56 (95%CI: 0.33-0.95), respectively. PFS at 36 months was 6.5% (95%CI: 0.0 - 17.0) in SCHOLAR-5, compared to 54.4% (95% CI: 44.2 - 63.5) in ZUMA-5. TTNT at 36 months was 45.7% (95%CI: 33.1 - 58.3) in SCHOLAR-5, compared to 59.5% (95%CI: 50.2 - 67.6) in ZUMA-5. OS at 36 months was 64.3% (95%CI: 52.2 - 76.4) in SCHOLAR-5, compared to 75.5% (95% CI: 66.9 - 82.2) in ZUMA-5.
Conclusion: After follow-up, axi-cel continues to demonstrate a substantial and statistically significant improvement in meaningful clinical endpoints including ORR, CR, PFS, TTNT and OS compared to currently available therapies for r/r FL patients, highlighting the durable treatment effect of axi-cel. These findings suggest that axi-cel addresses an important unmet medical need for r/r FL patients.
Disclosures
Ghione:AstraZeneca Pharmaceuticals: Consultancy; Kyowa Hakko Kirin: Consultancy; Kite Pharma: Research Funding; Secura Bio: Consultancy. Palomba:Garuda: Honoraria; Thymofox: Honoraria; Da Volterra: Honoraria; Vor Biopharma: Honoraria; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Frazier Healthcare Partners: Honoraria; Pluto Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Lygenesis: Honoraria; Ceramedix: Honoraria; Notch Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Nektar Therapeutics: Honoraria; Synthekine: Honoraria; BMS: Consultancy; Seres: Current holder of stock options in a privately-held company, Honoraria, Research Funding; Kite: Honoraria; GSK: Honoraria; Rheos: Honoraria; MustangBio: Honoraria; Novartis: Honoraria. Ray:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company. Limbrick-Oldfield:RainCity Analytics: Current Employment. Owen:Delta Hat: Current Employment. Kanters:RainCity Analytics: Research Funding. Jacobson:Clinical Care Options: Speakers Bureau; Nkarta: Consultancy, Honoraria; Humanigen: Consultancy, Honoraria, Other: Travel Support; Axis: Speakers Bureau; Ispen: Consultancy, Honoraria; ImmPACT Bio: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Celgene: Other: Travel Support; Daiichi Sankyo: Consultancy, Honoraria; Precision BioSciences: Consultancy, Honoraria, Other: Travel Support; Epizyme: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; Lonza: Consultancy, Honoraria, Other: Travel Support; Pfizer: Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel Support; BMS/Celgene: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding. Neelapu:Bio Ascend: Consultancy, Honoraria; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Bluebird Bio: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Other: Personal fees; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Medscape: Consultancy, Honoraria; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Aptitude Health: Consultancy, Research Funding; Poseida: Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.. Ghesquieres:Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria; BMS: Honoraria; Abbvie: Honoraria. Nahas:Kite, a Gilead company: Current Employment, Current holder of stock options in a privately-held company. Beygi:Kite, a Gilead Company: Current Employment, Research Funding. Patel:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company. Gribben:Janssen: Honoraria, Other: Grant and personal fees ; AbbVie: Honoraria, Other: Personal fees; Roche/Genentecg: Other: Personal fees; Celegene: Research Funding; AstraZeneca: Honoraria, Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Gilead/Kite: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal